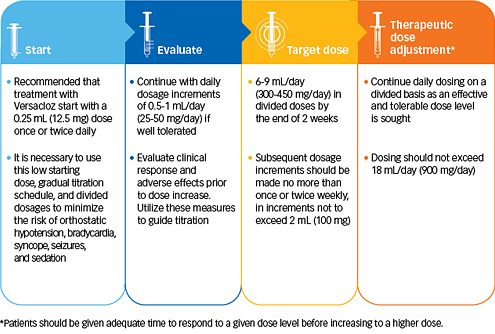
Initial treatment1
To start a new patient on Versacloz
Oral Suspension:
- Order lab: Write order for white blood cell (WBC) with differential or complete blood count (CBC) with differential
- Verify results: WBC ≥(3.5) 3500/mm3 and absolute neutrophil
count (ANC) ≥(2.0) 2000 mm3 - Write prescription
- Certify in the Clozapine REMS Program. Go to https://www.clozapinerems.com to start the prescriber certification process and identify a pharmacy certified to dispense clozapine products. The Clozapine REMS Program replaces the individual clozapine patient registries and the National Non-Rechallenge Master File (NNRMF). For additional information about the Clozapine REMS Program, please call 844-267-8678.
Important Safety Information
DRUG INTERACTIONS
- CYP1A2 Inhibitors: Reduce VERSACLOZ dose to one-third when coadministered with strong CYP1A2 inhibitors (e.g., fluvoxamine, ciprofloxacin, enoxacin). Dose reduction may be necessary with concomitant use of moderate or weak CYP1A2 inhibitors (e.g., oral contraceptives or caffeine).
- CYP2D6 or CYP3A4 Inhibitors. Dose reduction may be necessary with concomitant use of CYP2D6 or CYP3A4 inhibitors (e.g., cimetidine, escitalopram, erythromycin, paroxetine, bupropion, fluoxetine, quinidine, duloxetine, terbinafine, or sertraline).
- CYP1A2 or CYP3A4 Inducers. Concomitant use with strong CYP3A4 inducers (carbamazepine, phenytoin, St. John's wort, rifampin) is not recommended. Dose increase may be necessary with concomitant use of moderate CYP1A2 (e.g., tobacco smoke) or CYP3A4 inducers. Consider reducing VERSACLOZ dose when CYP1A2 (e.g., tobacco smoke) or CYP3A4 (e.g., carbamazepine) inducers are discontinued.
Click here for additional safety information.
Reference: 1. Versacloz® Prescribing Information, Douglas Pharmaceuticals America Ltd. 2025
The information on this website is intended for use only by residents of the
United States.
United States.